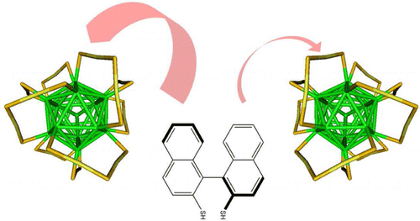-
Isolation of atomically precise mixed ligand shell PdAu24 clusters

A. Sels, N. Barrabés, S. Knoppe and T. Bürgi
Nanoscale, 8 (21) (2016), p11130-11135


DOI:10.1039/C6NR00931J | unige:84474 | Abstract | Article HTML | Article PDF | Supporting Info
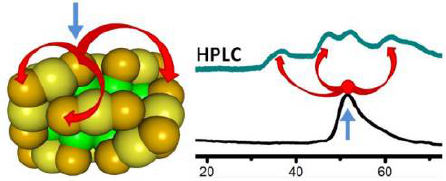
Exposure of PdAu24(2-PET)18 (2-PET: 2-phenylethylthiolate) to BINAS (1,1-binaphthyl-2,2-dithiol) leads to species of composition PdAu24(2-PET)18â2x(BINAS)x due to ligand exchange reactions. The BINAS adsorbs in a specific mode that bridges the apex and one core site of two adjacent S(R)âAuâS(R)âAuâS(R) units. Species with different compositions of the ligand shell can be separated by HPLC. Furthermore, site isomers can be separated. For the cluster with exactly one BINAS in its ligand shell only one isomer is expected due to the symmetry of the cluster, which is confirmed by High-Performance Liquid Chromatography (HPLC). Addition of a second BINAS to the ligand shell leads to several isomers. In total six distinguishable isomers are possible for PdAu24(2-PET)14(BINAS)2 including two pairs of enantiomers concerning the adsorption pattern. At least four distinctive isomers are separated by HPLC. Calculations indicate that one of the six possibilities is energetically disfavoured. Interestingly, diastereomers, which have an enantiomeric relationship concerning the adsorption pattern of chiral BINAS, have significantly different stabilities. The relative intensity of the observed peaks in the HPLC does not reflect the statistical weight of the different isomers. This shows, as supported by the calculations, that the first adsorbed BINAS molecule influences the adsorption of the second incoming BINAS ligand. In addition, experiments with the corresponding Pt doped gold cluster reveal qualitatively the same behaviour, however with slightly different relative abundances of the corresponding isomers. This finding points towards the influence of electronic effects on the isomer distribution. Even for clusters containing more than two BINAS ligands a limited number of isomers were found, which is in contrast to the corresponding situation for monothiols, where the number of possible isomers is much larger.